About Us
To save more lives with lab tests
To lead the healthcare industry in rapid, clinically meaningful, innovative laboratory solutions
Integrity, Quality, Solutions-Oriented, Constantly Improving Service
Delivering high-quality services with the fastest-in-the-nation speed
Specialized Lab and CRO
Trusted Expertise
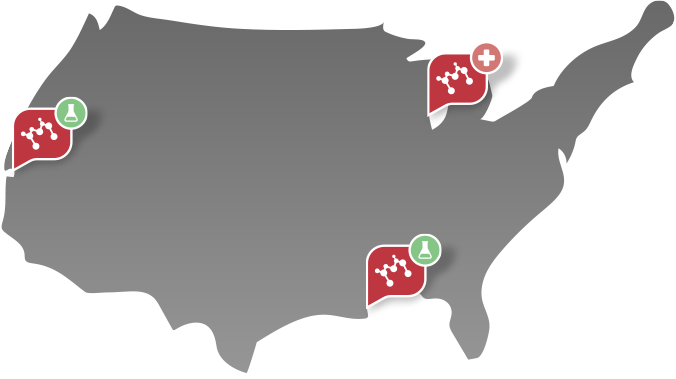
Machaon Diagnostics is a CAP, CLIA-accredited specialized reference laboratory and contract research organization (CRO). We are experts in coagulation, platelets, complement, genetics and rare disease. We have laboratories located in Berkeley, California and New Orleans, Louisiana.

Scope
- Coagulation & Platelet Disorders
- Complement-mediated Diseases
- Immunology
- Rare Diseases
- Kidney Diseases
- Comprehensive Genetics
Staff
- 80% of staff are MD, PhD, CLS, MS or professionally licensed
- 600+ years of aggregate specialized laboratory experience
- 50% of CLS staff are trained CAP laboratory inspectors

9 out of 10 Top Medical Centers Choose Machaon
CRO and Testings United Under One Umbrella
The majority of premier medical centers (according to US News) rely on Machaon Diagnostics to provide critical diagnostic services.

Why Machaon?
- Usually the Fastest STAT TAT in the Industry
- Expansive Specialized Tests
- Lab Testing and CRO Services United Under One Umbrella
- Customer First Approach
Who Else is Choosing Machaon?
- 8 out of the 10 largest global pharmaceutical companies
- 8 out of the 10 largest global CROs

In this episode we hear an interview with Mike Ero, Machaon Diagnostics’ Founder and CEO. A Clinical Laboratory Scientist (CLS) who added an MBA, Mike started Machaon Diagnostics back in 2003 at the age of 29. Just how did all of that come about? Listen and find out.

MICHAEL ERO
Unparalleled Service
Savings More Lives with Lab Tests
We have rapidly grown into a group of pathologists, scientists, consultants and technologists dedicated to saving lives through testing. Collectively, the Machaon Diagnostics team brings over 600 years of combined expertise to the field of laboratory medicine.

Certification
- CAP & CLIA-accredited
- Multi-state licensed
- Minority Business Enterprise (MBE)
- GCLP-certified staff
Compliance
- CAP, CLIA & State Compliant SOPs
- GCP & GLP-compliant Study Design
- GDPR-compliant Trial Design
- IVDR Projects
Papilio Machaon
Papilio Machaon (Old World Swallowtail Butterfly)
Over the last 20 years we have transformed from an esoteric coagulation laboratory to advanced hemostasis, complement, genetics and rare disease Lab/CRO
Our Goal: Fastest in the Industry
with high quality results
The faster a physician can get a lab result, the better it is for the patient. This golden rule of lab medicine is often the difference between life and death.
Machaon Diagnostics only offers a test if we can consistently achieve the fastest STAT TAT in the industry.
Workflow
- TAT performance shared with clients
- Test progression monitoring
- Realtime courier monitoring
- 95% TAT confidence intervals measured
Support
- SMS text alerts on critical lab results
- Critical results called (with verbal read back)
- Genetic counseling
- MD/CLS consultations
Machaon Highlights
- Founded in 2003 by a clinical laboratory scientist (CLS)
- 2 CAP/CLIA sites
- 80% of staff are MD, PhD, CLS, MS or professionally licensed
- 50% of our CLS staff are trained CAP inspectors
- CA medical director is a key opinion leader in TMAs, benign hematology disorders and kidney diseases
- Louisiana medical director is a nationally recognized pathology expert in thrombosis and hemostasis
- Existing relationships with hospital systems in 50 states and 20 countries
- Clients include small, medium and large pharma, biotech, CROs for local to global studies
Our Milestones